Hydrogen is the most abundant element in the known universe. On earth, the vast majority of hydrogen atoms are part of molecules such as natural gas (primarily methane, CH4) or water (H2O). Almost no pure hydrogen molecules (H2) occur naturally – and none of them are green or blue! Pure molecular hydrogen is a colourless, non-toxic gas. It’s also the subject of much discussion as a carrier of low-carbon energy.
The terms ‘blue hydrogen’ and ‘green hydrogen’ are commonly used in relation to hydrogen production methods, discussed below.
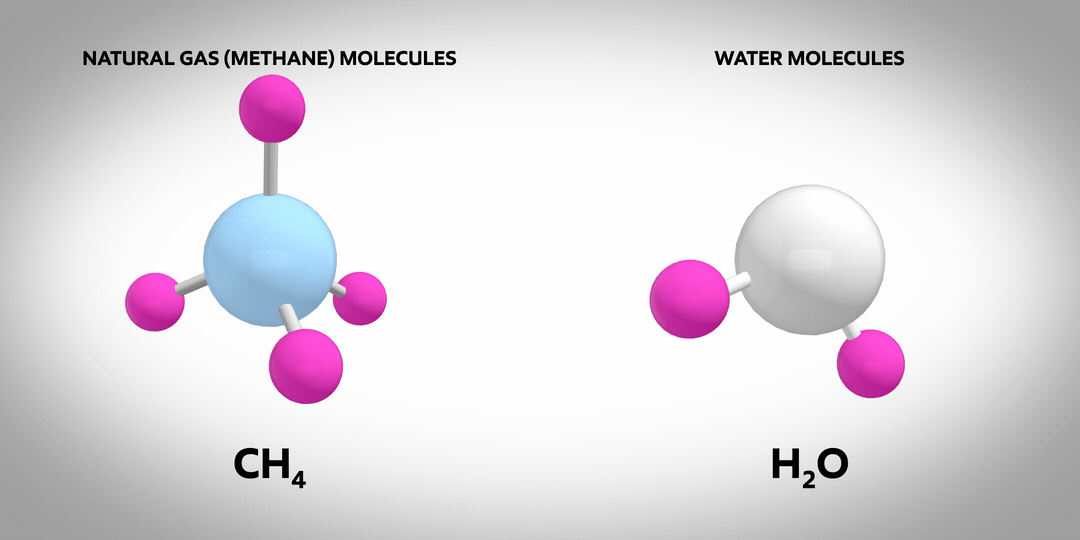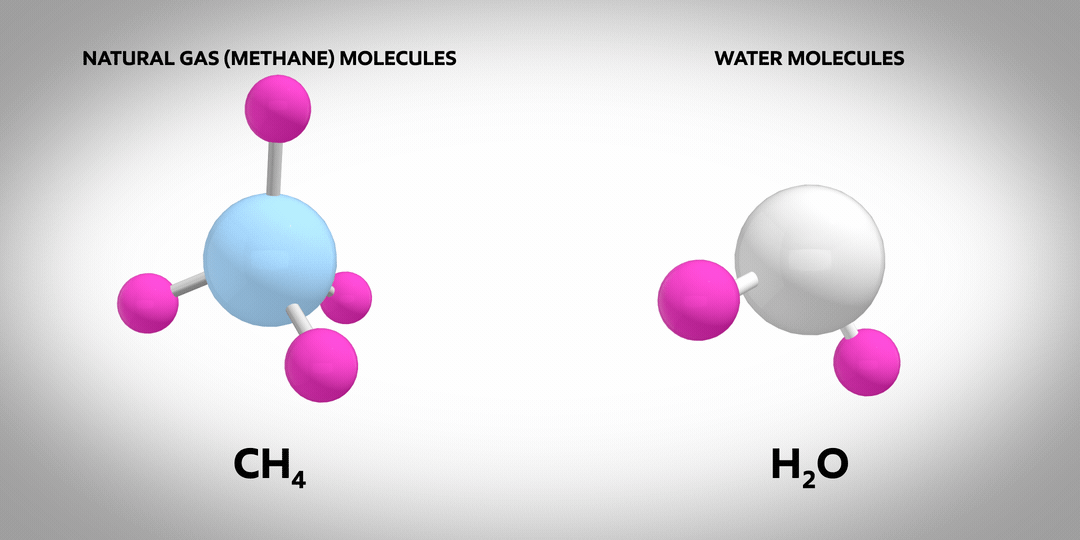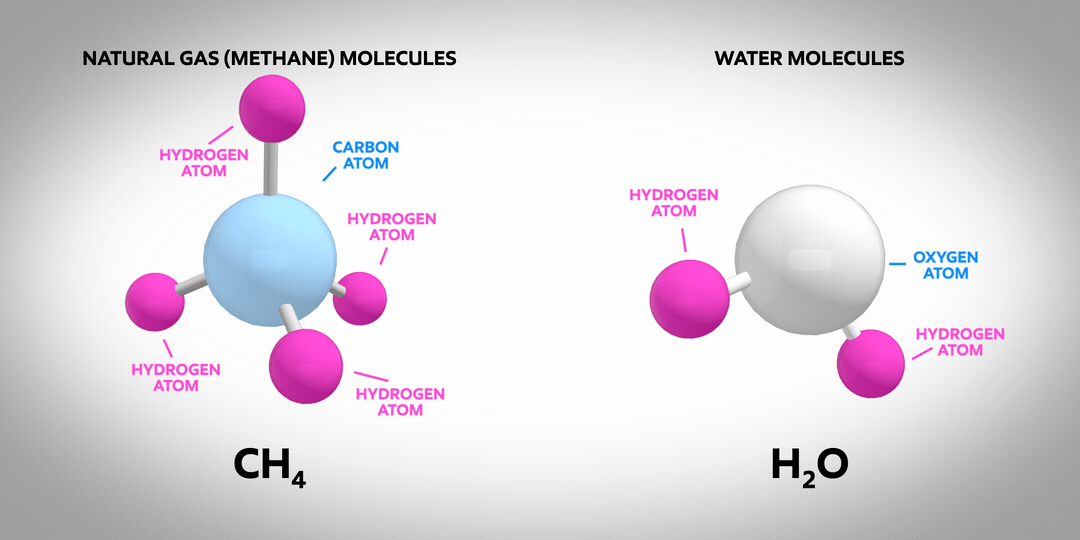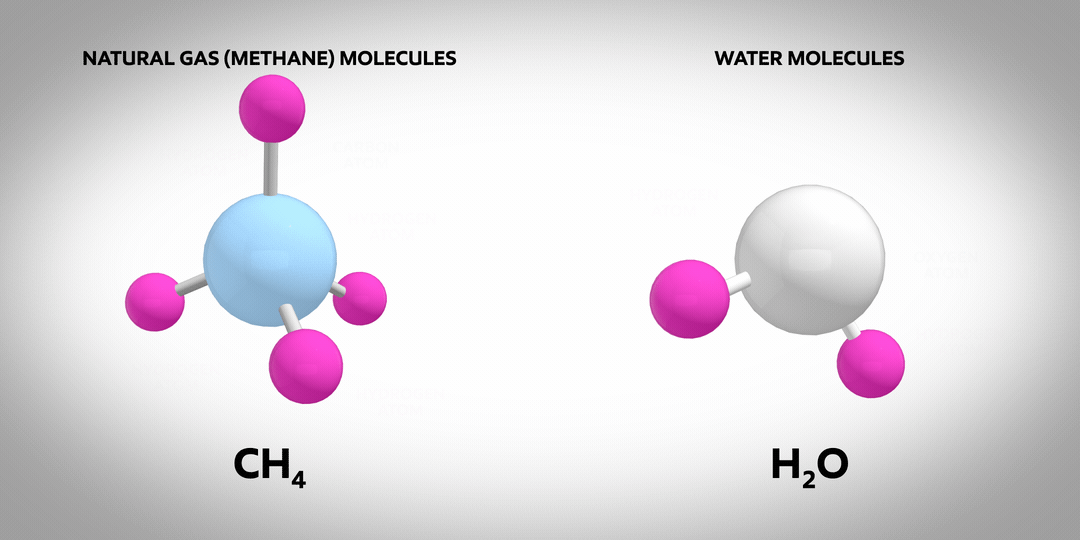
Once manufactured, hydrogen can be used to power vehicles or produce the high temperature heat needed for industrial manufacturing processes. It emits no CO2 when burned, which makes it an attractive energy carrier for lowering carbon emissions.
Making Low-Carbon H2: What are ‘Blue Hydrogen’ and ‘Green Hydrogen’?
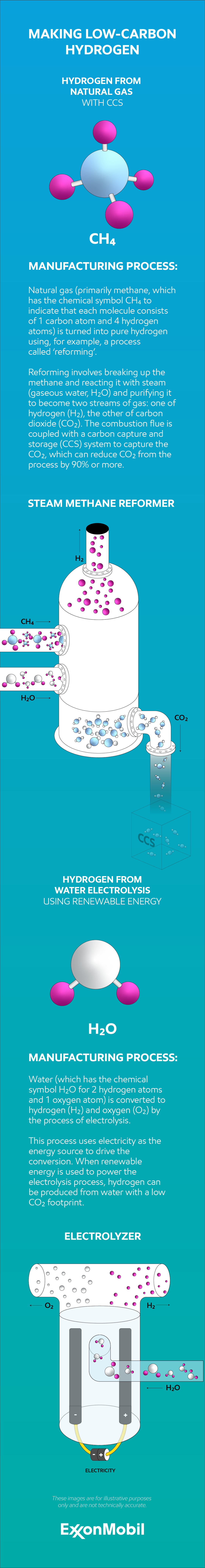
Hydrogen can be made from several abundant sources – such as natural gas or water – and during that process, energy is required. The process and energy used determine whether the hydrogen produced is ‘low-carbon’ or not.
Hydrogen made from natural gas must incorporate carbon capture, utilization and storage (CCUS) into the process to be low-carbon. This process is one way of making what is commonly referred to as ‘blue hydrogen’.
When hydrogen is made from water via electrolysis, the process must be powered exclusively by a low-carbon source such as renewable energy to be a low-carbon process. This process is commonly referred to as ‘green hydrogen’.
Although they are frequently-used terms, the labels ‘blue hydrogen’ and ‘green hydrogen’ have no commonly agreed-on definition. Some might describe any hydrogen production process that utilizes CCUS as ‘blue hydrogen’, even when natural gas isn’t the feedstock. Others might call any production process that utilizes electrolysis ‘green hydrogen’, even if it uses grid power that would result in an increase in CO2 overuse of natural gas.
Whatever they are labeled, both hydrogen from natural gas with CCUS and from water using renewable energy have a role to play as part of a lower-carbon future. We’ve put together this infographic to explain the key differences between the two processes:
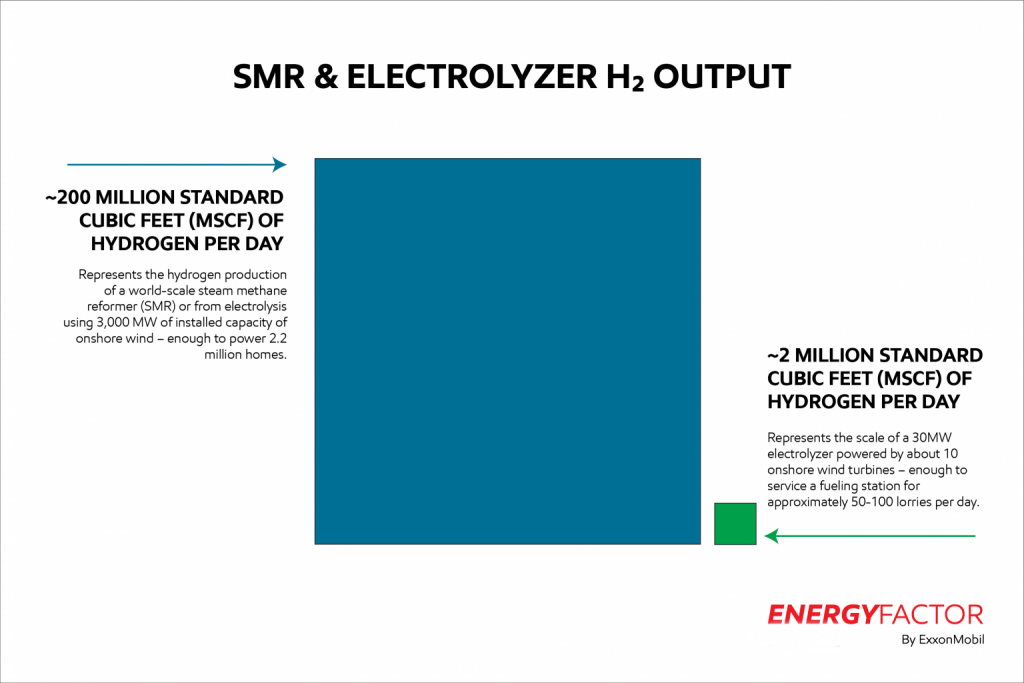
The Scale of Hydrogen Production
Today’s natural gas reforming technology lends itself to the industrial manufacture of hydrogen on a large scale. A world-class methane reformer might produce 200 million standard cubic feet (MSCF) of hydrogen per day. That’s enough hydrogen to support an industrial cluster or refuel 10,000 lorries.
Current hydrogen electrolyzers are smaller scale. A unit comprised of 30 MW of electrolyzers powered by 10 onshore wind turbines can produce 2 MSCF of hydrogen per day, lending itself to more decentralized applications where not as much hydrogen is required.
With the deployment of CO2 policy and hydrogen incentives, demand for hydrogen will rise and its use will gain traction – requiring both production technologies for hydrogen.
The Versatility of Hydrogen
As long as it is low-carbon, hydrogen is a versatile clean-burning fuel with huge potential.
Follow ExxonMobil’s progress in developing carbon capture and storage technology and hydrogen.