This article was originally published in 2016. M. Stanley Whittingham was awarded the Nobel Prize in Chemistry on October 9, 2019 for the development of the rechargeable battery and the following has been updated to reflect this news.
If you are reading this on a phone, tablet or laptop, you should probably thank Dr. M. Stanley Whittingham. In the 1970s Dr. Whittingham was working at ExxonMobil’s Clinton, New Jersey, corporate research lab when he created the very first examples of a radical new technology: the rechargeable lithium-ion battery.
For this groundbreaking work, Dr. Whittingham was awarded the 2019 Nobel Prize in Chemistry along with Dr. John Goodenough of the University of Texas at Austin and Dr. Akira Yoshino of Meijo University in Nagoya, Japan. Today, Whittingham is a Distinguished Professor of Chemistry and Materials Science at Binghamton University in New York.
Prior to joining Binghamton, Dr. Whittingham worked at ExxonMobil, where his research paved the way for the development of the rechargeable lithium-ion battery. Specifically, he and his team discovered that when lithium ions were held between plates of titanium sulfide, the ions could move back and forth between the positive and negative contacts, creating electricity.
With lithium-ion batteries, “we have gained access to a technical revolution,” said Sara Snogerup Linse, a professor of physical chemistry at Lund University in Sweden who chairs the Nobel committee for the chemistry prize.
Rechargeable batteries had been around for decades when Whittingham first proposed his version. But the rechargeable batteries at that time were bulky lead-acid cells – the kind still found in many cars today. And although the disposable carbon zinc batteries that power your remote control were prevalent, replacing them after each charge of a more energy-hungry device like a computer would be both annoying and expensive.
Early research had suggested that the highly reactive metal lithium could be used to store energy, but Dr. Whittingham was the first to figure out how to make it happen at room temperature without the risk of explosion. His original design used titanium sulfide, a 2.5-volt material, and the intercalation design (or insertion of ions in a removable way) was sound and provided the basis for modern lithium-ion batteries.

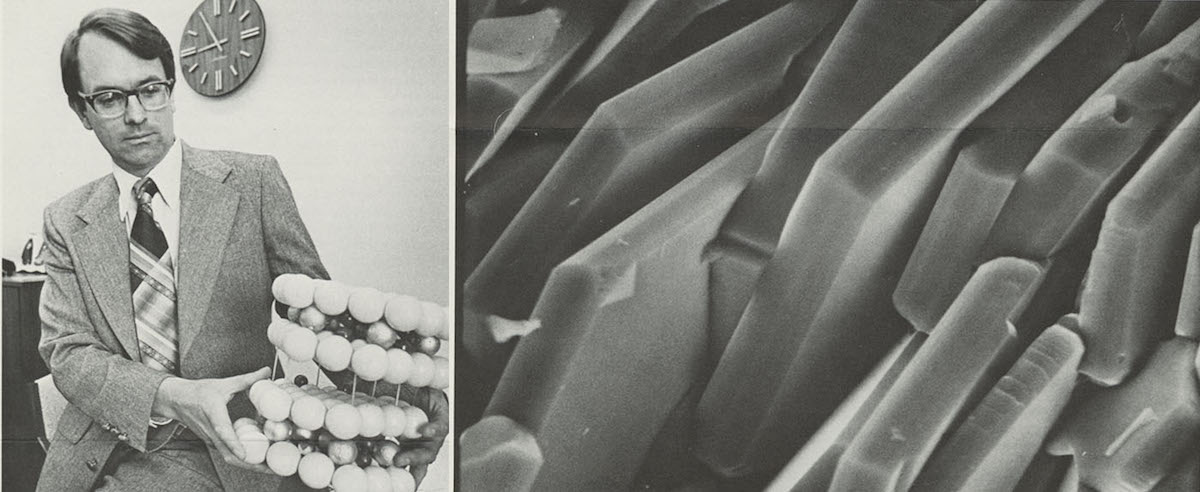
Dr. Whittingham in 1979 in an Exxon Research and Engineering publication. Right, Dr. Whittingham today as a professor at Binghamton State University. Image credits: ExxonMobil, Dr. M. Stanley Whittingham
In 1980, Dr. Whittingham worked with fellow Nobel laureate Dr. Goodenough at the University of Texas in Austin to improve his initial breakthrough by using metal oxides and higher, 4-volt materials. Building on that work on the other side of the world in Japan, Dr. Yoshino was able to develop the first commercial lithium-ion battery.
The high-energy-density lithium-ion technology now powers laptops, tablets, cellphones and most electric cars. It even allows solar-powered aircraft like the record-setting Solar Impulse 2 to continue flying after the sun has set. Power grids that are based on inconsistent power sources like wind or solar power are also beginning to rely on massive lithium-ion batteries to store energy for times when demand exceeds output.
Dr. Whittingham, Dr. Goodenough and Dr. Yoshino built on each other’s work and turned breakthrough research into an innovation that’s transformed how the world uses and stores energy.
Image credit: ExxonMobil Historical Collection, [identifier number: di_10643-di_10650], The Dolph Briscoe Center for American History, The University of Texas at Austin
Sources:
Forbes: Solar Plane Takes To The Skies Again To Display Clean Energy’s Potential
Pipe Dream: BU professor recognized for contributions to creation of lithium-ion battery
Quartz: The man who brought us the lithium-ion battery at the age of 57 has an idea for a new one at 92